Protection without poison
Published:
Aaron Match developed a new theory for why the ozone layer is up in the stratosphere, reaching a maximum 26 km above the surface in paper submitted to the journal of Atmospheric Chemistry and Physics. In 1880, Walter Hartley deduced that ozone must be absorbing UV-B and UV-C radiation from the sun, but since it isn’t present at the surface (except in polluted air), this ozone must be somewhere “up there”. The stratosphere wasn’t discovered for a couple more decades, but it turns out that ozone that shields us from this harmful UV radiation is largely between 16 and 40 km above us. It’s a good thing its there: ozone is toxic. If it were uniformly distributed through the atmosphere, the concentration at the surface would be 8 times the EPA safety limit. Why is ozone safely up in the stratosphere, where it protects us without poisoning us? Advanced chemistry climate models can accurately predict the distribution of ozone, but in turns out our text book understanding of the ozone layer were incomplete and gave the wrong explanation for why it reaches a maximum in the stratosphere at 26 km.
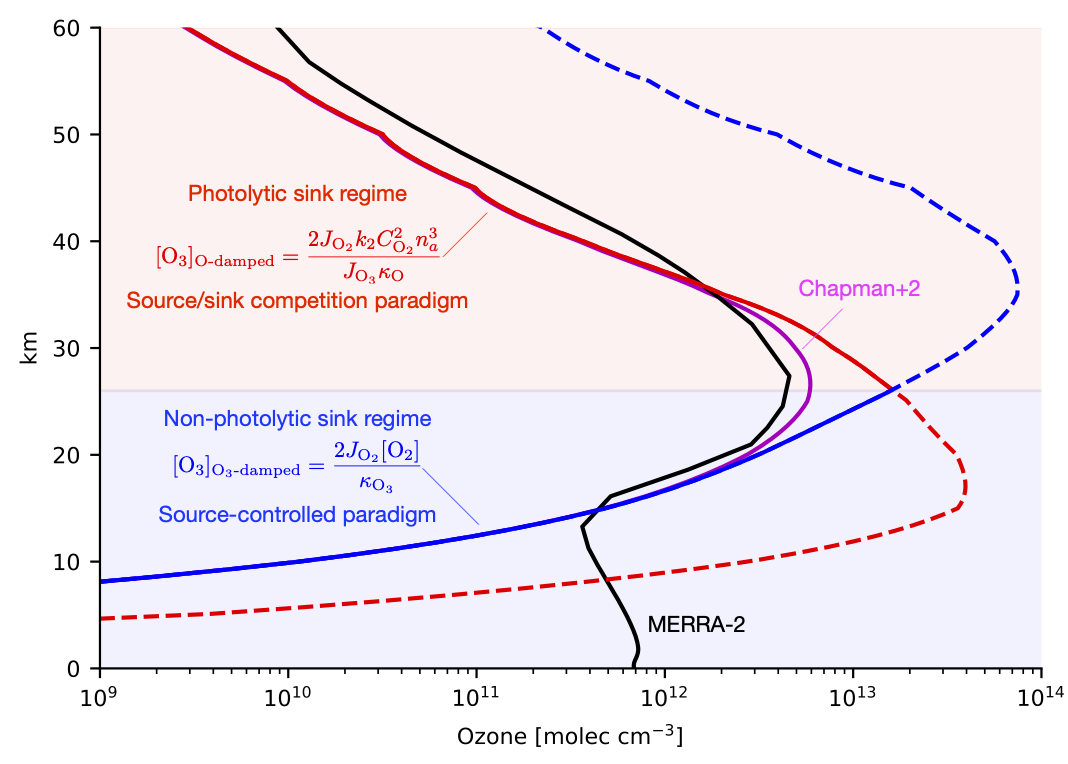
The image above is admittedly complicated, but captures the heart of Aaron’s argument. He developed a modified version of the Chapman Cycle, which we dub the “Chapman +2” model as it includes two additional generalized reactions, to show how a regime transition from a photolytic sink regime above 26 km to a non-photolytic sink regime below 26 km most accurately explains why ozone reaches a maximum precisely at this transition point. The new theory has implications for how the ozone layer should respond to perturbation. Check out the paper to find out!